Sulfidic mine tailings are usually featured by deficient organic matter and nutrients, weak water holding and penetrating capacity, low abundance of microbes, low pH, and high contents of toxic elements. These characteristics inhibit plant establishment and thereby result in the dispersion of toxic elements.
Compost- and irrigation-assisted phytostabilization is an eco-friendly and sustainable technique to establish a vegetative cover that inhibits the offsite transport of toxic elements in tailing. However, the compost solution and redox oscillations may affect the biogeochemical behavior of As, due to its high affinity to Fe minerals and dissolved organic matter.
Recently, a joint study by Prof. LIU Chengshuai's group from the Institute of Geochemistry of the Chinese Academy of Sciences (IGCAS) and Jon Chorover's group from The University of Arizona has revealed the effect of compost solution on As release in mine tailings under biogeochemical redox oscillations.
The study was published in Geochimica et Cosmochimica Acta on Sept. 16.
The researchers designed a redox-stat reactor system for tailings to react with compost solution or an equivalent ionic strength electrolyte for seven redox cycles, with each cycle including 7 d anoxic and 7 d oxic. They analyzed the aqueous chemistry, mineralogy, and solid phase speciation of As and Fe during and after oscillations.
Results showed that compost solution significantly increased the mobilization of As under anoxic environment. The addition of compost solution also stimulated the oxidation of residual pyrite in tailings, resulting in a drop in pH and an increase in sulfate.
The release of As was dominated by the reductive dissolution of ferrihydrite and microbially-mediated As reduction. However, the As released in the anoxic half-cycle was repartitioned to the solid phase concurrent with the precipitation of ferrihydrite and jarosite in the subsequent oxic half-cycle.
During the anoxic half-cycle, ferrihydrite was preferentially reduced, while during the oxic half-cycle, the high sulfate, ferrous iron, and hydronium activity promoted the precipitation of jarosite, which sequestered As in structure. Therefore, the crystallinity of Fe oxides increased and the mobilization of As decreased with increasing redox cycles.
The study provides a scientific basis for the remediation assessment and environmental risk assessment of mine site compost-assisted phytostabilization.
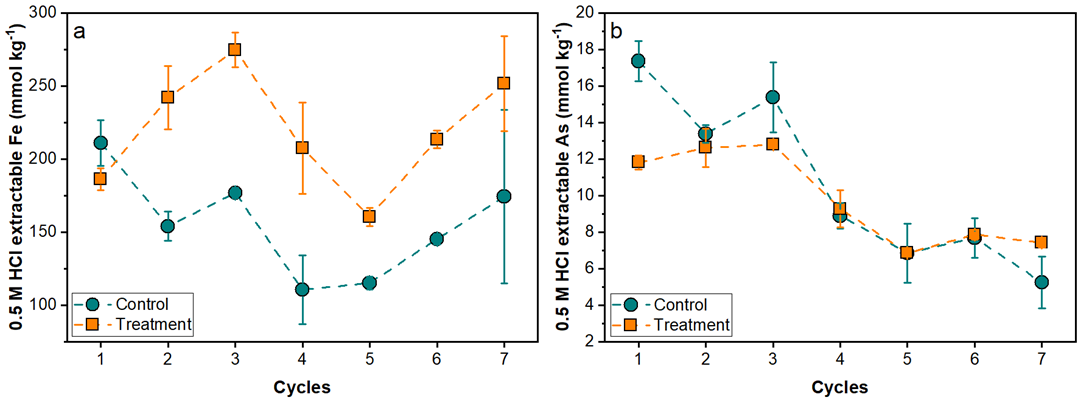
Fig. 1 The 0.5 M HCl extractable Fe (a) and As (b) from tailing solids after each redox full cycle. Extractable As decreases with increased cycling under control and compost treatment. (Image by IGCAS)
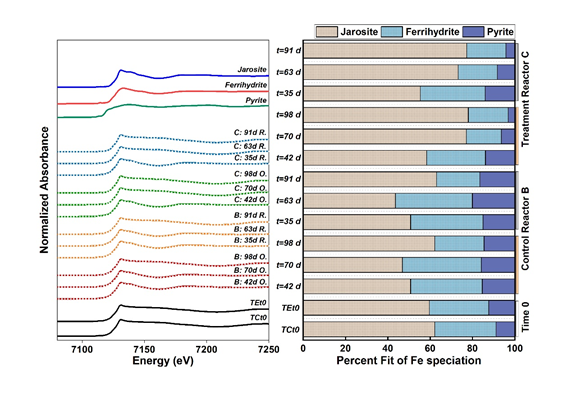
Fig. 2 Linear combination fits of Fe K-edge XANES showing percent components in samples from Control (B) and compost Treatment (C) through time. (Image by IGCAS)
Contact:
Dr. LIU Yizhang
Institute of Geochemistry, Chinese Academy of Sciences
Email: liuyizhang@mail.gyig.ac.cn
(By Prof. LIU Chengshuai' s Group)