Stable carbon isotope composition (δ13C) of carbonates has been widely used to implicate paleoclimate and paleoecology.
However, results from field observations and laboratory experiments have shown that the δ13C difference between calcite and solution can vary up to 3‰ even under a similar set of solution composition and temperature, raising uncertainties on implications for paleoclimate and paleoecology.
Researchers from the Institute of Geochemistry of the Chinese Academy of Sciences (IGCAS) reveal that the different thicknesses of the stagnant liquid layer between solid and well mixed bulk solution (i.e., diffusion boundary layer, DBL) may cause the large δ13C difference between calcite and solution in nature.
The study was published in Earth and Planetary Science Letters on April 14.
The scientists designed a clever natural experiment to investigate the carbon isotopic and elemental compositions of a natural travertine deposit (Baishuitai, SW China) consisting of meter-scale travertine terraces.
The results showed that the δ13C and Mg/Ca ratios were higher while Sr/Ca ratios were lower for the pool-bottom calcites than for the rim calcites.
By applying a mass transfer model, the scientists quantitatively interpreted the differences in carbon isotope and elemental compositions of the abiogenic calcites by the different hydrodynamic conditions.
The inverse variation in Mg/Ca and Sr/Ca ratios in calcites arose from the different precipitation rates between at the pool-bottom and at the rim, while the consistently lower δ13C for calcites at the rim was due to their higher pH at the solid-solution interface than for calcites precipitated at the pool-bottom.
Generally, stiller waters had thicker DBLs and result in lower pH on the mineral surface, which drove the apparent carbon isotope fractionation values between calcite and dissolved inorganic carbon (DIC) higher.
This study, for the first time, provides a mechanistic interpretation on the variability of the observed carbon isotope fractionation between calcite and DIC in riverine or cave environments.
"This work solves a current dilemma in the interpretation of carbon isotopes from natural samples and experimental precipitates," said Prof. Chris Romanek from Furman University.
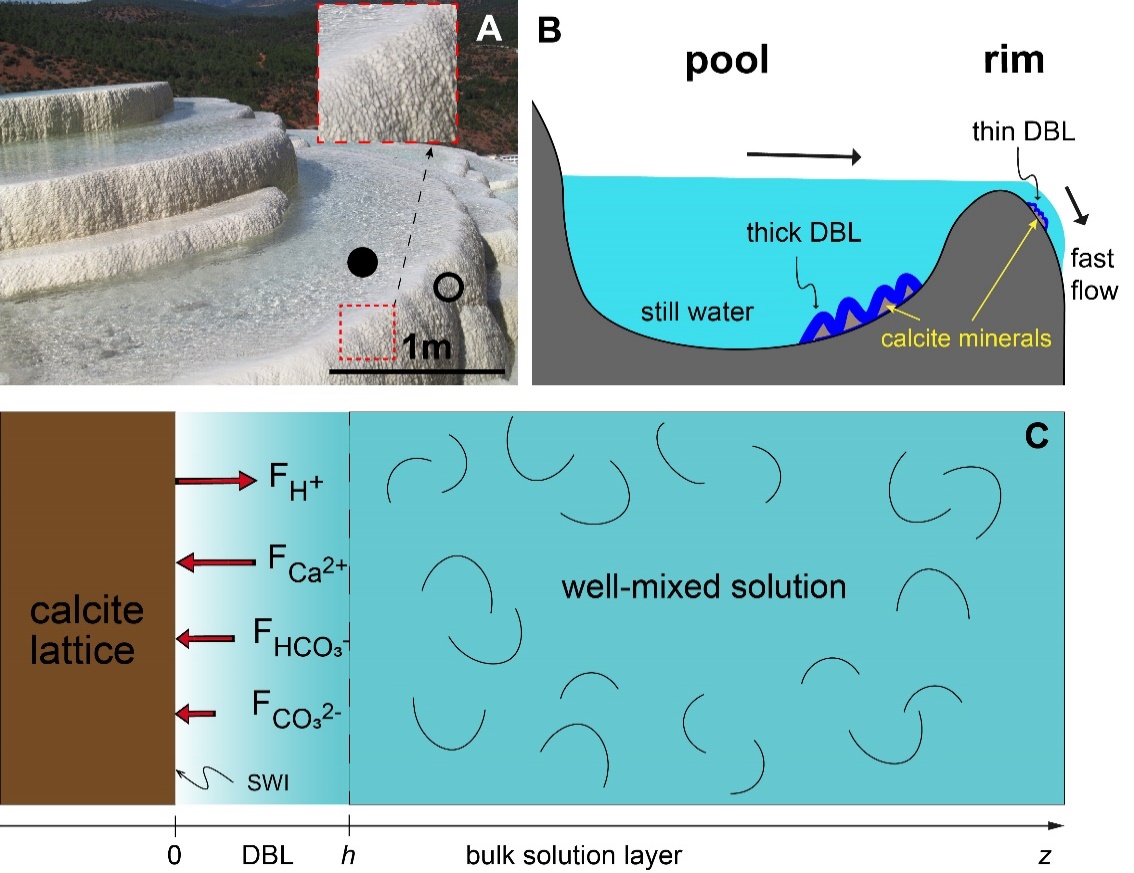
Fig. 1. Sketch of hydrodynamic controls on the thickness of diffusion boundary layer (DBL) on the surface of calcite minerals. (Image by IGCAS)
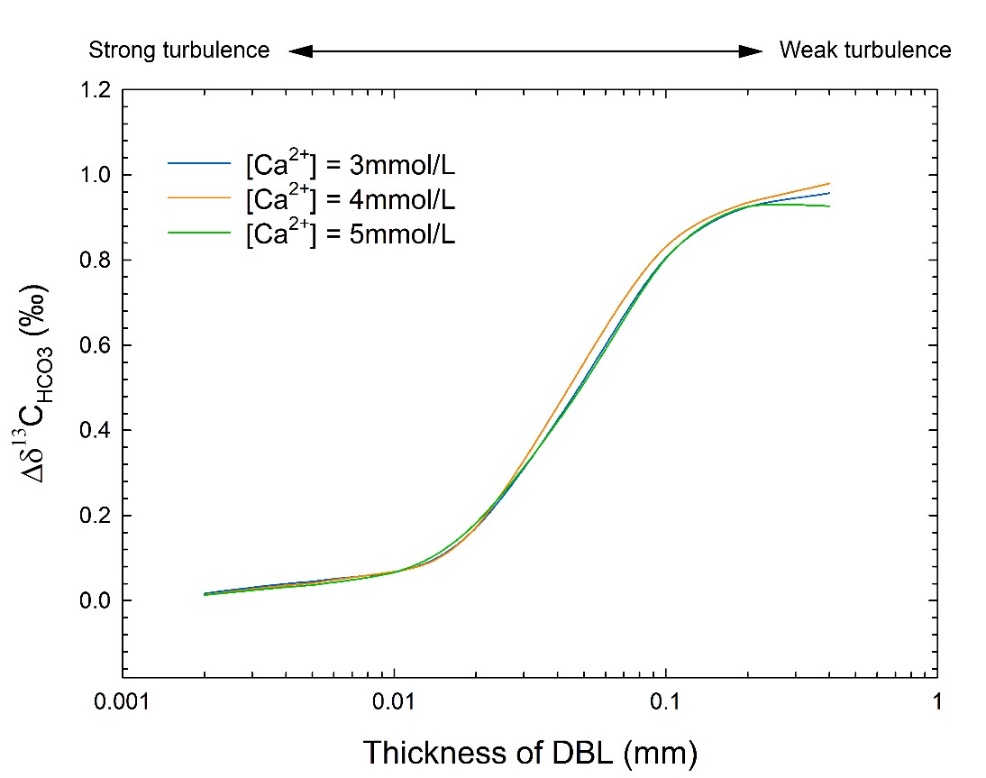
Fig. 2. The δ13CHCO3 difference between at the mineral-solution interface and in the bulk solution (δ13CHCO3) as a function of DBL thickness under the conditions of T=20 °C, pH=8.3, and various concentrations of Ca2+. (Image by IGCAS)
Contact: YAN Hao; LIU Zaihua
Institute of Geochemistry, Chinese Academy of Sciences
E-mail: yanhao@mail.gyig.ac.cn; liuzaihua@mail.gyig.ac.cn
(By Professor LIU Zaihua’s group)