With the rapid development in mass spectrometry techniques, Mg isotope has been applied to many earth science investigations, such as the reconstruction of paleo-environments and the weathering of silicates.
However, recent experimental and theoretical studies reported significantly different results in predicting Mg isotope fractionations between minerals and solutions.
A research team led by Prof. LIU Yun from the Institute of Geochemistry of the Chinese Academy of Sciences (IGCAS) used volume variable cluster model (VVCM), a newly designed method, to investigate equilibrium Mg isotope fractionations between Mg-bearing minerals and solutions.
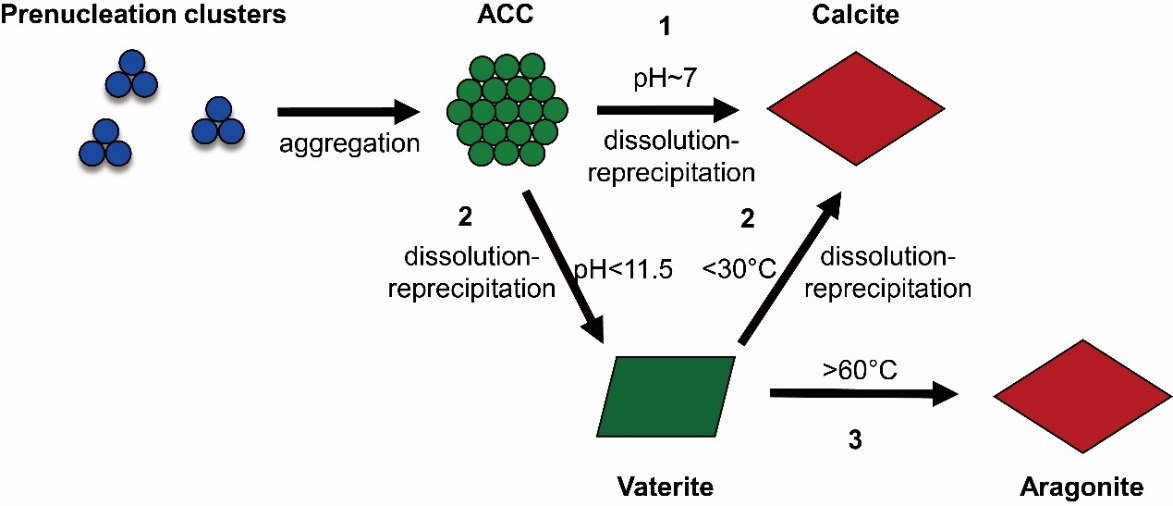
The researchers found that the discrepancies between theoretical and experimental works for Mg isotope fractionation between carbonate minerals and solutions might be caused by several factors including the incorporation of hydrated Mg2+ into the solids, the concentration effect, and the emergence of intermediate precursors, e.g. amorphous calcium carbonates (ACCs), which suggested that the processes investigated by experimental and theoretical works might not be the same one.
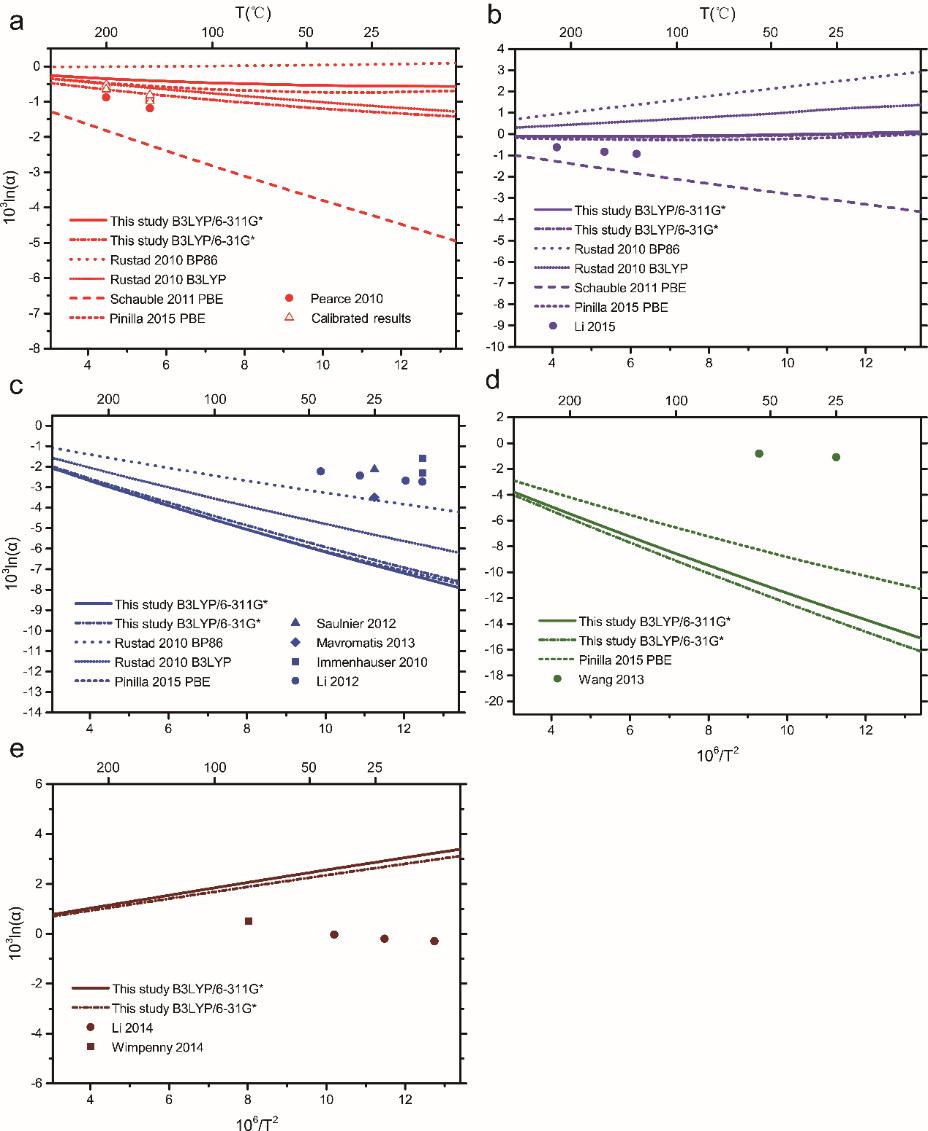
The results also showed that the Mg isotope fractionation between brucite and aqueous Mg2+ are predicted as +2.7‰ and +2.9‰ at B3LYP/6-31G* and B3LYP/6-311G* levels, respectively.
Relative to coexisting aqueous Mg2+, ACCs were enriched with heavy Mg isotopes. Combining the results of previous works, an average value of +1.45‰ was obtained for the fractionation between ACCs and aqueous Mg2+ at room temperatures.
The study provides a base to understand the accumulating Mg isotope data. It was published in Chemical Geology.
|
|
|
Schematic representation of non-classical crystallization pathways of carbonate minerals. (Image by IGCAS)
|
|
|
| Theoretical predictions of Mg isotope fractionations between minerals and aqueous Mg2+. (a) Magnesite case. (b) Dolomite case. (c) Calcite case. (d) Aragonite case. (e) Brucite case. (Image by IGCAS) |
Contact
LIU Yun
The Institute of Geochemistry of the Chinese Academy of Sciences
E-mail: liuyun@vip.gyig.ac.cn
(By GAO Caihong)