Apatite is a group of phosphate minerals, usually referring to hydroxyapatite, fluorapatite, and chlorapatite, named for high concentrations of OH−, F− or Cl− ions, respectively. It is well known as one of the common components of terrestrial and lunar rocks as well as of meteorites, and contains significant amounts of rare earth elements (REE) and large ion lithophile elements (LILE). As naturally occurring apatites contain large amounts of the rare earth elements, it has been well known that apatite controls a large portion of the budget of these trace elements. In addition, apatites were stable at pressures and temperature conditions correspond to upper mantle of the Earth. Therefore, apatites are an important carrier of REE, LILE, and the volatile elements to the upper mantle of the Earth.
Prof. ZHOU Wenge’s team at Institute of Geochemistry, CAS (IGCAS) has investigated the high-pressure behavior of a synthetic rare-earth silicate apatite-- Ca4La6(SiO4)6(OH)2 using in situ angle-dispersive x-ray diffraction and a diamond anvil cell up to 9.33 GPa. In their research, no phase change of Ca4La6(SiO4)6(OH)2 was observed up to 9.33 GPa, because as pressure increased, all the peaks shifted continuously toward higher 2θ and the diffraction lines broaden and weaken slightly, but the overall pattern did not change. The values of zero-pressure volume V0, K0, and K'0 refined with a third-order Birch-Murnaghan equation of state are V0=579.2±0.1 Å3, K0=89±2 GPa, and K'0=10.9±0.8. If K'0 is fixed at 4, K0 is obtained as 110±2 GPa. Analysis of axial compressible modulus shows that the a-axis (Ka0 = 79±2 GPa) is more compressible than the c-axis (Kc0 = 121±7 GPa).
Prof. ZHOU Wenge’s team also shows a comparison of this study and the previous studies for calcium apatites at room temperature, and find that the K0 value of 110 GPa obtained in this study for Ca4La6(SiO4)6(OH)2 is substantially larger (by about 12%) than the values of calcium apatites when K'0 is 4. Finally, the differences in the elastic behavior of Ca4La6(SiO4)6(OH)2 and calcium apatites can be mainly attributed to the substitution by large cations (La) at the Ca(2) sites of apatites.
Prof. ZHOU Wenge's and Dr. FAN Dawei's research were supported by the National Natural Science Foundation of China (Grant No. 41004035, 41374107 and 41274105), Western doctor special fund of the West Light Foundation of The Chinese Academy of Sciences (2011, to Fan Dawei), and more results have been published in Phys. Chem. Minerals 2014, 41, 85.
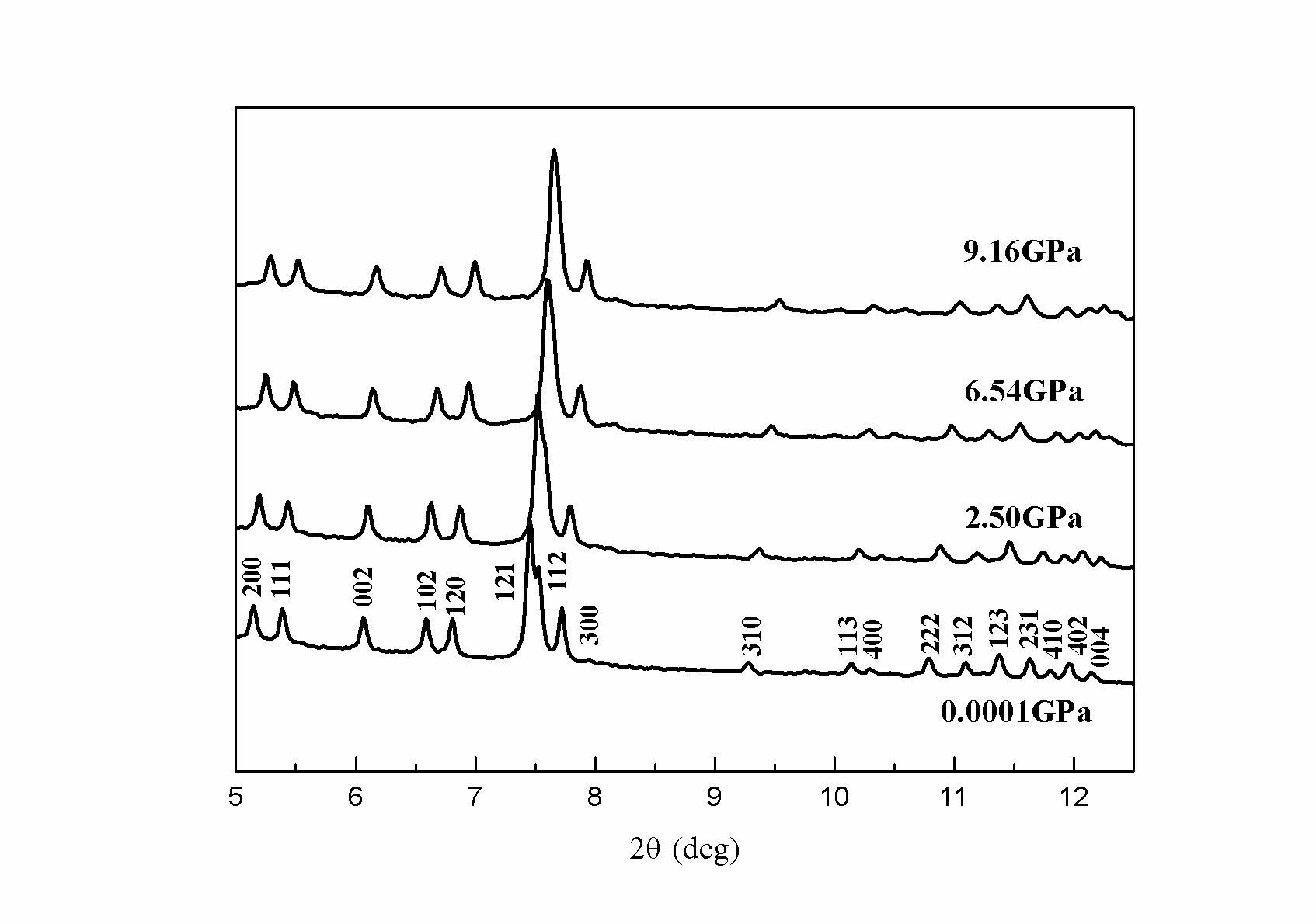 |
| Fig. Representative X-ray diffraction patterns of Ca4La6(SiO4)6(OH)2up to 9.33 GPa (Image by IGCAS) |
(By FAN Dawei)