 |
 |
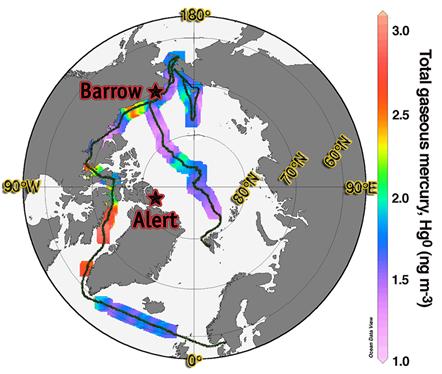
| FigureThe spatial distribution of Hg0 in air (upper panel) and dissolved in surface water (lower panel) along the cruise track (Adopted from (Sommar et al., 2010) and (Andersson et al., 2008c) respectively). |
The Arctic is no longer a pristine environment, free of anthropogenic contaminants such as persistent organic substances, heavy metals or radionuclides. Though it is remote, the Arctic is surrounded by populated continents to which it is well connected by the currents of atmosphere and oceans. Concerning the neurotoxic element mercury (Hg), it has been elucidated that high levels in the Arctic environment is related to a rapid, near-compete depletion of Hg
0 (MDE) in the marine boundary-layer occurring episodically during the Polar spring. Unlike O
3, which is chemically destroyed during the catalytic process involving reactive bromine species, Hg only changes its oxidation state (Hg
0→Hg
II), and depletion from the air may imply accumulation in the polar environment (Sommar et al., 2007). However, the fate of surplus mercury deposited to the Arctic basin during polar spring MDEs is largely indefinite with reference to transport and transformation during the following summer.
After joining Prof. FENG Xinbin’s group in Institute of Geochemistry, Chinese Academy of Science (IGCAS), Dr. Jonas Sommar has been involved in a series of publications (Andersson et al., 2011; Andersson et al., 2008c; Sommar et al., 2010) based on samples collected during a nearly three month long expedition to the high Arctic during the summer of 2005. Using the Swedish icebreaker Oden as a platform, measurements of Hg0 in air and dissolved in surface sea water was performed continuously with high time-resolution (10 min) along a route over the North Atlantic and the Arctic Ocean. The expedition was the first time that these species have been measured over the Arctic Ocean going from 60° to 90°N. A novel in-house built apparatus relying on air-water equilibrium was employed to facilitate a high frequency in Hg0(aq) measurements (Andersson et al., 2008a).
In general, Hg0 levels in the high Arctic exceed those from more temperate marine background regions during summer. Hg0 concentrations in ambient air were at an average ~10% higher while concerning the corresponding Hg0(aq)-data, there is a more striking positive difference with the adjacent Atlantic and Pacific Oceans. The spatial distribution of Hg0 levels in air and dissolved in oceanic surface water (in units of ng m-3 and fM respectively) along the route is shown in the Figure. It includes some striking features. For example, once the ship entered the ice (in the Baffin Bay), Hg0(aq) increased drastically by over 350% while Hg0(g) became strongly variable with peaks above 4 ng m-3. The degree of saturation of Hg0 (kH•[Hg0(aq)]/[Hg0(g), where kH is the Henry’s law coefficient for Hg0(Andersson et al., 2008b)) in the Arctic Ocean was observed to be as high as 1800% (average 440%). Hence, it is concluded that HgII deposited to the marine environment during MDEs is to a significant degree reduced back to Hg0 resulting in re-cycling of mercury to the atmosphere during the summer. The sea-ice acts as a partial barrier for the exchange process, thus permitting high levels of Hg0(aq) to build up in the surface water beneath it. In terms of an abrupt future warming, the current cycling of Hg in the Arctic will be largely perturbed and sea-air interaction will be even more important in its atmospheric behaviour.
References
Andersson, M. E., Sommar, J., Gårdfeldt, K., and Jutterström, S., 2011. Air-sea exchange of volatile mercury in the North Atlantic Ocean. Marine Chemistry 125, 1-7.
Sommar, J., Andersson, M. E., and Jacobi, H. W., 2010. Circumpolar measurements of speciated mercury, ozone and carbon monoxide in the boundary layer of the Arctic Ocean. Atmosheric Physics & Chemistry 10, 5031-5045.
Andersson, M. E., Gårdfeldt, K., and Wängberg, I., 2008a. A description of an automatic continuous equilibrium system for the measurement of dissolved gaseous mercury. Analytical and Bioanalytical Chemistry 391, 2277-2282.
Andersson, M. E., Gårdfeldt, K., Wängberg, I., and Strömberg, D., 2008b. Determination of Henry's law constant for elemental mercury. Chemosphere 73, 587-592.
Andersson, M. E., Sommar, J., Gårdfeldt, K., and Lindqvist, O., 2008c. Enhanced concentrations of dissolved gaseous mercury in the surface waters of the Arctic Ocean. Marine Chemistry 110, 190-194.
Sommar, J., Wängberg, I., Berg, T., Gårdfeldt, K., Munthe, J., Richter, A., Urba, A., Wittrock, F., and Schroeder, W. H., 2007. Circumpolar transport and air-surface exchange of atmospheric mercury at Ny-Ålesund (79oN), Svalbard, spring 2002. Atmosheric Physics & Chemistry 7, 151-166.
(By Jonas Sommar)