Although Se isotopes are widely treated as research objects in the field of nontraditional stable isotope, there is a serious problem: the lack of Se isotope fractionation parameters has severely limited our ability to explain further the large number of data obtained from experiments. So far, a few equilibrium Se isotope fractionation factors among the gaseous Se-containing compounds are provided by Krouse and Thode (1962) using the measured normal vibrational spectra. In addition, Schauble (2004) calculated the isotope systems based on the spectroscopic data using the empirical force field method.
Recently, LI XueFang, from Prof. LIU Yun's group at the State Key Laboratory of Ore Deposit Geochemistry (SKLODG) of Institue of Geochemistry, Chinese Academy of Sciences (IGCAS) calculated several equilibrium Se isotope fractionation parameters in gaseous, aqueous and condensed phases (Earth and Planetary Science Letters Volume 304, Issues 1-2, 1 April 2011, Pages 113-120). Using Density Functional Theory (at the B3LYP/6-311+G(d,p) level), they accurately calculated the parameters among more than ten Se-bearing species at different temperatures. Their results show that Se isotope fractionation almost entirely depends on the valence states: the higher valence state, the heavier isotopes. Moreover, a trend of the enrichment of heavy Se isotopes is suggested as SeO42- > SeO32- > HSeO3- > SeO2 > selenoamino acids > alkylselenides > Se(0) or H2Se > HSe-. Natural Se(0) can slightly enrich heavy isotopes compare with H2Se.
Assuming the absence of reduction of bacteria and in pure inorganic conditions, the equilibrium fractionation diagram in the gaseous and aqueous cycling is shown in the following picture (25℃):
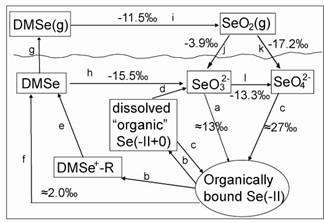
Figure Equilibrium Se isotope fractionation diagram in biogeochemical cycle of selenium based on Fig. 6 in the paper of Cooke and Bruland (1987). (a) reductive assimilation of selenate and selenite into organically bound selenide by organisms; (b) release of organically bound selenide back to solution; (c) assimilation of dissolved organic selenide by organisms; (d) oxidation of dissolved organic selenide to selenite; (e) conversion of dissolved DMSe+-R to dimethyl selenide (DMSe); (f) direct release of DMSe to solution; (g) degassing of DMSe to the atmosphere; (h) oxidation of DMSe to selenite; (i) oxidation of DMSe to SeO2 in atmosphere; (j) conversion of gaseous SeO2 to selenite; (k) conversion of gaseous SeO2 to selenate; and (l) oxidation of selenite to selenate. (Image by IGCAS)
The fundamental work of LI XueFang and LIU Yun will effectively propel the development of Se isotope geochemistry.
(Provided by LI Xuefang)