MAO Xumei*, TIAN Xike, and YU Chengyong
School of Environmental Studies and School of Material Sciences and Chemical Engineering, China University of Geosciences, Wuhan 430074, China
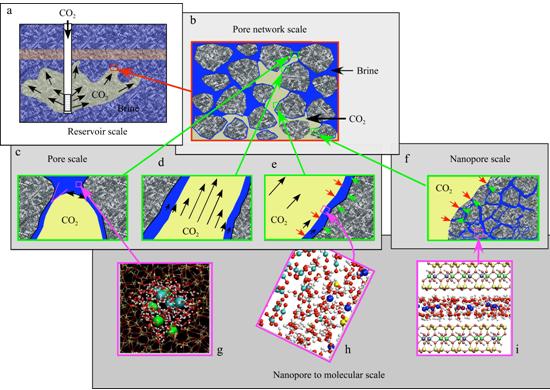
Fig. CO2 fluid/rock interaction in the process of geologic storage. a. Distribution of CO2 on a reservoir scale; b. CO2 diffusion through pore networks; c. capillary breakthrough by pore-scale process; d. multi-phase flow; e. diffusive exchange and reaction across the CO2-H2O interfaces; f–i. CO2 fluid/rock interaction in nanopores and molecular-scale processes. (Tokunaga and Wan, 2001)
Abstract The increase of CO2 in atmosphere is a main factor leading to “greenhouse effect”, which causes more and more serious global environmental problems. The reduction of CO2 is a challenge for the survival of human beings, and it is also a big technical problem. CO2 fluid-rock interaction is a key scientific problem involved in geological storage. The CO2 fluid-rock interaction has a variety of multi-scale changes. Due to great differences in the quantity of surface atoms and surface energy between micron-nano-sized minerals, and ions and crystals, the speed and efficiency of CO2 fluid-rock interaction on a micron-nano scale are much higher than those on other scales. As is known from the natural world, the micron-nano structures of pores and the surface chemical modification of natural porous minerals (zeolite, diatomite, sepiolite, palygorskite, halloysite, etc.) should be further investigated, which can be used as the micron-nano -mineral porous materials with high capacity and high efficiency for capturing CO2. Through simulating the adsorption capacity and process of CO2 by minerals in the natural world, the micron-nano technology is applied to calcium- and magnesium-based minerals (olivine, pyroxene, feldspar, clay, etc.) so as to improve the activity of calcium and magnesium and enlarge the reaction contact area. In this way, the efficiency of capturing and storage of CO2 by calcium- and magnesium-based minerals can be greatly improved. These minerals can also be used as the micron-nano-mineral materials with large capacity and high efficiency for capturing and storing CO2.
Key words micron-nano mineral; CO2; capturing and storage; fluid-rock interaction
* Corresponding author, E-mail: maoxumei@cug.edu.cn
CHINESE JOURNAL OF GEOCHEMISTRY Vol. 30, No. 4, 2011, page 569
© Science Press and Institute of Geochemistry, CAS and Springer-Verlag Berlin Heidelberg 2011