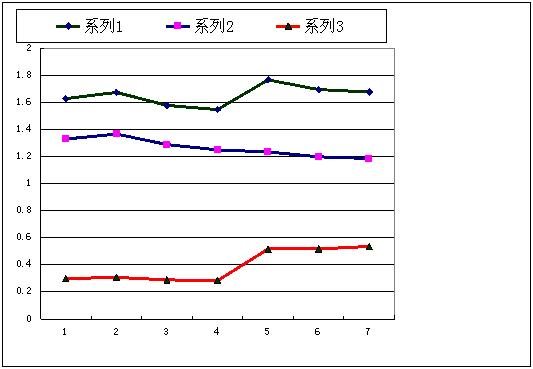
Fig. 7. Bond length of Si-O, ionic radii of oxygen and cation radii of silicon in magnesium silicate and oxides under difference pressures.
SHI Ni-cheng, Li Guo-wu, MA Zhe-sheng, XIONG Ming
(Crystal structure Laboratory of University of Geosciences, Beijing 100083, China)
Abstract: In this paper the study on crystal chemical behavior of silicon and oxygen ions in Mg2SiO4 and MgSiO3 under high pressures were continued. Ionic radii of oxygen and silicon in magnesium silicate and magnesium-silicon oxides, i.e. olivine, ringwoodite, and the pases of Mg2SiO4, MgSiO3 (perovskite and post perovskite) under high pressures were investigated. The result indicated that ionic radius of oxygen decreased gradually with increasing pressure, but cation radius of silicon increased and phase transitions occurred with increasing pressure. Radii of silicon cation of SiO2 polymorphs also increased with phase transition under high pressures. This variation may be result of a transition from ionic compounds to metallic compounds under high pressures.
Key words: high-pressure; phase transition; crystal chemistry; coordination polyhedron; ionic radius; perovskite; post-perovskite
Corresponding author: SHI Ni-cheng, Email: snc2011@126.com
ACTA MINERALOGICA SINICA Vol. 30, No. 4, 2010, Page 411-416