The element Gallium (Ga) has chemical properties similar to aluminum (Al), and is frequently used as a geochemical analog of Al. However, unlike Al which is monoisotopic element, Ga has two stable isotopes, 69Ga and 71Ga, with the abundances of 60.1% and 39.9% respectively.
Ga isotopes ratio, which reveals large variation of δ71Ga (up to 1.83‰), could be beneficial for the studies of both the biogeochemical cycle of Ga and the geochemical behaviors of Al. Adsorption on oxides, clays, carbonates, and inorganic and organic colloids is known to be an important process in surficial environments for controlling the biogeochemical behaviors of trivalent elements like Ga.
A research team led by Prof. CHEN Jiubin from the Institute of Geochemistry of the Chinese Academy of Sciences (IGCAS) carried out for the first time a laboratory experiment of Ga isotope fractionation during adsorption on calcite and goethite.
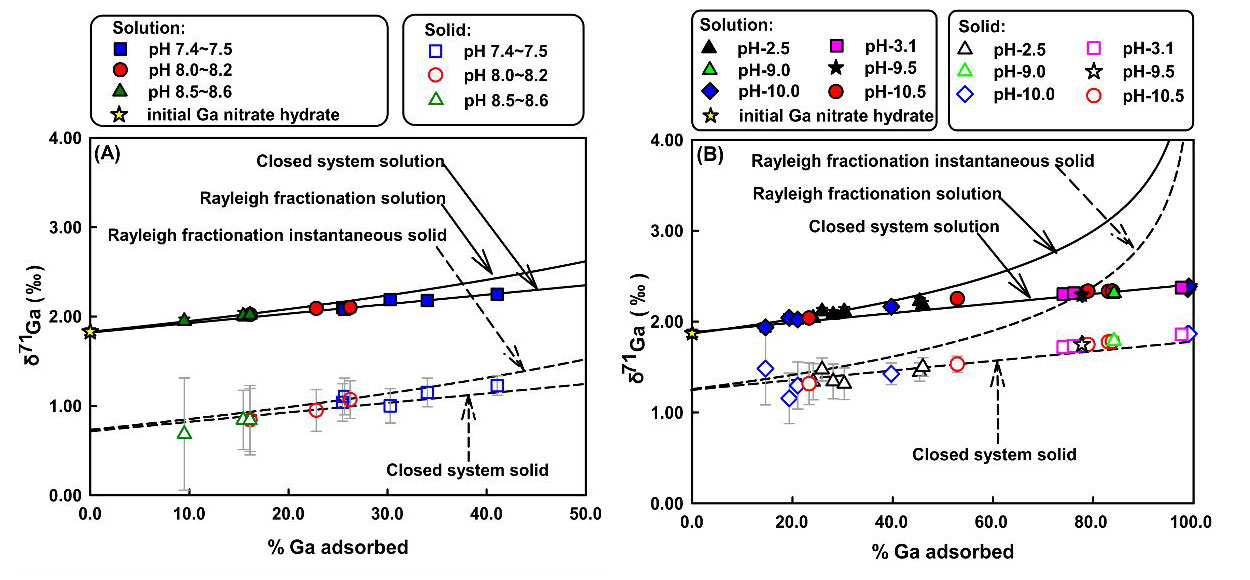
In all experiments, the lighter Ga isotopes were preferentially adsorbed onto the solid surface, with isotope fractionation between solid and solution △71Ga solid-solution up to -1.27‰ and -0.89‰ for calcite and goethite respectively (Figure). The results suggested that the large fractionation was likely caused by the change of Ga speciation from the solution to the newly formed Ga complex.
For calcite, Ga isotope fractionation is triggered by increased Ga coordination and Ga–O bond length, from Ga(OH)4- in solution to >Ca–O–GaOH(OH2)4+ in surface complex. While for goethite, despite the formation of Ga hexa-coordinated >FeOGa(OH)20 complexes both at acid and alkaline pH, a similar extent of isotope fractionation is found at acid and alkaline pH due to the fact of preferentially adsorption of Ga(OH)4- for all investigated condition.
The results could be extended to the adsorption of Ga by oxides, carbonates or clay minerals. Therefore, significant Ga isotope fractionation would occur between primary phases and secondary minerals and/or surficial fluids such as seawater. Ga isotopes are thus useful tools for better characterizing the surficial biogeochemical cycles of Ga and its geochemical analog Al.
The paper entitled "Gallium isotope fractionation during Ga adsorption on calcite and goethite" was published in Geochimica et Cosmochimica Acta.
|
|
| Fig. δ71Gasolutionand δ71Gacalcite(both measured and inferred) as a function of the fraction of Ga adsorbed by calcite (A) / goethite (B). (Image by IGCAS) |
Contact:
CHEN Jiubin
Institute of Geochemistry, Chinese Academy of Sciences
E-mail: chenjiubin@vip.gyig.ac.cn
(By Prof. CHEN Jiubin's group)