As one of the most important physicochemical processes in the Earth system, diffusion plays a key role in the different scale of chemical exchange and the transfer of matter. Quantitative determination of elemental diffusion coefficients of minerals and rocks, especially the diffusion coefficient as a function of temperature, pressure, composition, and so on, are crucial for our understanding many geodynamical processes in the Earth's interior and the evolution of Earth.
Researchers from the Key Laboratory for High-Temperature and High-Pressure Study of the Earth's Interior, Institute of Geochemistry, Chinese Academy of Sciences (IGCAS), has made a first application of a thermodynamic model ( so-called cBΩ model) to the geophysical and geochemical fields. On the basis of the available elastic data and equation of state of solids, a novel method of calculation of diffusion coefficient has been established for the different elements in various minerals (Zhang et al., 2010, 2011; Zhang, 2012; Zhang and Wu, 2011, 2013; Zhang, 2014).
In their recent study, Dr. ZHANG Baohua and Dr. SHAN Shuangming from IGCAS applied such a thermodynamic approach to predict Si self-diffusion and Fe-Mg interdiffusion in the silicate minerals (i.e., Olivine, Wadsleyite, Ringwoodite, Garnet and Perovskite). The interdiffusion coefficient of Fe–Mg in (Fe, Mg)2SiO4 polymorphs and perovskite was calculated as a function of temperature and pressure. This calculation was conducted using a thermodynamic model that interconnects point defect parameters with bulk properties, i.e., the isothermal bulk modulus B, thermal expansion coefficient b, and atomic volume X. The calculated diffusivities of Si and Fe-Mg as a function of temperature and pressure from the cBΩ model are in good agreement with those determined by experimental and theoretical studies in most cases, as well as other diffusion parameters (i.e., activation enthalpy, activation entropy and activation volume). The excellent agreement between the calculated diffusion parameters and the experimental ones indicated that a thermodynamic model can quantitatively describe Fe–Mg interdiffusivity in silicate minerals over a broad range of temperatures and pressures.
The researchers' successful results can not only be useful in enriching the diffusion database and in planning the diffusion experiments in the future, but also provide important scientific evidences and methods for the further geophysical and geochemical applications.
The results have been published in Geochemistry Geophysics Geosystems and Journal of Applied Physics, respectively.
References:
1) Zhang Baohua and Shan Shuangming (2015) Application of the cBΩ model to the calculation of diffusion parameters of Si in silicates. Geochemistry Geophysics Geosystems 16(3), 705?718.
2) Zhang Baohua and Shan Shuangming (2015) Thermodynamic calculations of Fe–Mg interdiffusion in (Mg,Fe)2SiO4 polymorphs and perovskite. Journal of Applied Physics 117(5), 054906 (2015).
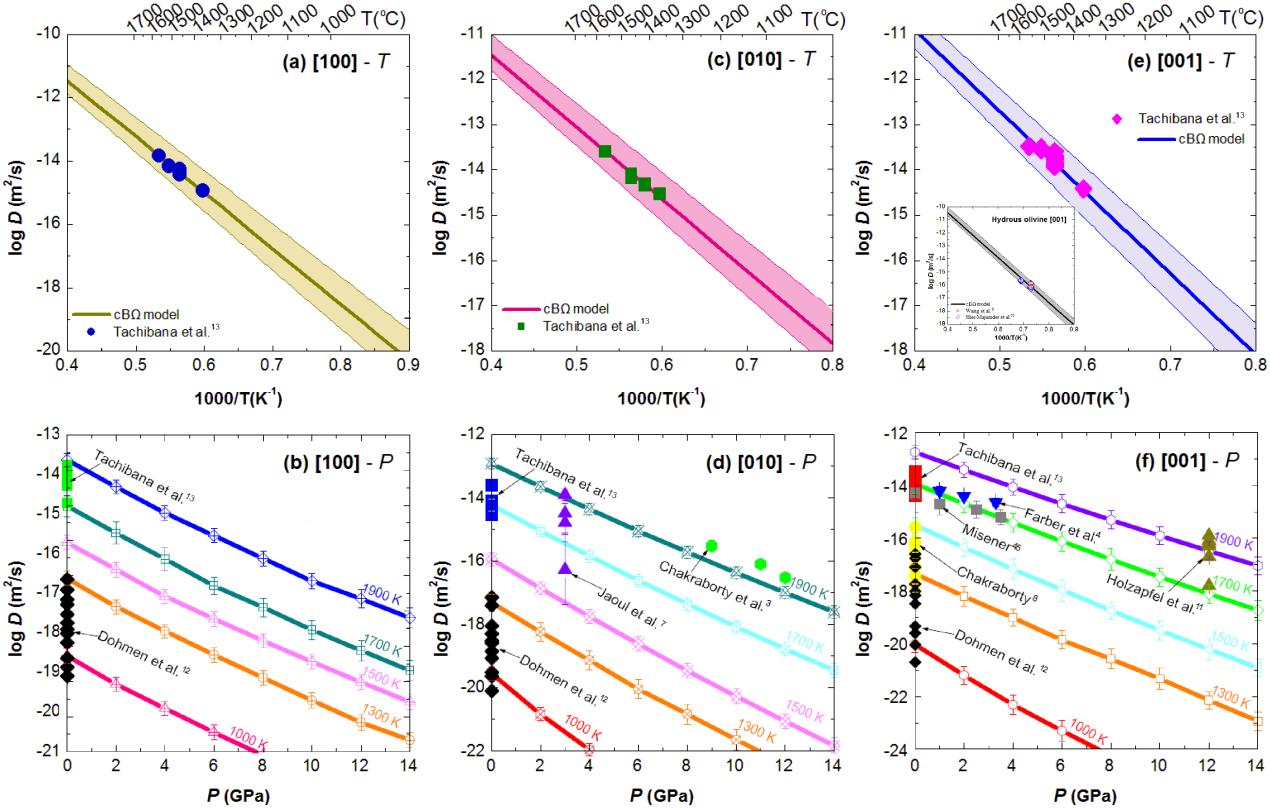 |
| Fig. Comparison of the calculated results derived from cBΩ model with the experimental data for Fe–Mg interdiffusion in anhydrous olivine. (Image by IGCAS) |
Contact:
ZHANG Baohua
Institute of Geochemistry, Chinese Academy of Sciences
E-mail: zhangbaohua@vip.gyig.ac.cn
(By Zhang Baohua)