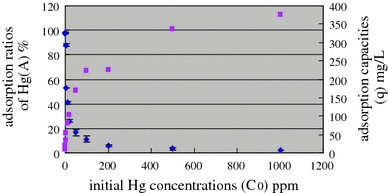
Fig. Changes of Hg adsorption ratios A (filled diamond) and adsorption capacities q (filled square) at different initial Hg concentrations (volume of the bacterial culture was 5 ml, total volume was 100 ml, pH were at around 6)
Our previous findings have indicated that Bacillus mucilaginosus might be a promising biosorbent. However, up to now, few studies have been performed to examine the use of B. mucilaginosus as a sorbent, especially as a sorbent for Hg(II). The aim of the current study was to investigate the adsorption of Hg(II) by B. mucilaginosus and the underlying mechanism involved. The results showed that B. mucilaginosus exhibited effective adsorption of Hg(II), and the experimental data were well fitted by the Langmuir model with equilibrium constant of 3.32 x 10(4) M-1 and maximum adsorption capacity of 393 mg(Hg)/l(bacterial culture). The average saturated adsorption amount of Hg(II) by each cell was 9.83 x 10(9) atoms, with time to reach adsorption equilibrium less than 10 min. The adsorption efficiency was mainly dependent on pH. Surface adsorption of capsules was identified to be the major mechanism for the biosorption of Hg(II) by B. mucilaginosus, which might be associated with the cell products on the surface of capsules of B. mucilaginosus. Differences observed in adsorption behaviors at different concentrations of Hg(II) were well explained using the Visual minTEQ software. Our findings might shed some lights on the application of B. mucilaginosus as an adsorbent for Hg(II) and other heavy metals.
| Publication name |
WORLD JOURNAL OF MICROBIOLOGY & BIOTECHNOLOGY Volume: 27 Issue: 5 Pages: 1063-1070 Published: MAY 2011 |
| Author(s) |
Mo, Bin-Bin; Lian, Bin |
| Corresponding author |
LIAN Bin
bin2368@vip.163.com
Chinese Acad Sci, State Key Lab Environm Geochem, Inst Geochem, Guanshui Rd 46, Guiyang 550002, Guizhou Prov Peoples R China |