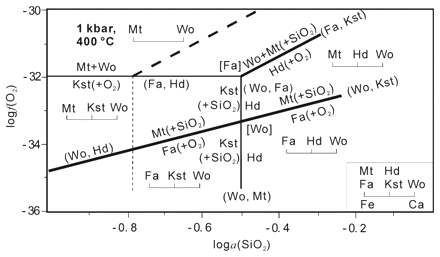
FIGURE Corrected logf(O2)-loga(SiO2) diagram of the Fe-Ca-Si-O system at 400 °C and 1 kbar. The insertion in the bottom corner is the projection of the chemographic diagram of the Fe-Ca-Si-O system from O2 and SiO2.
Some errors and metastable phase relations are found in a recently reported oxygen fugacity silica activity [logf(O2)-loga(SiO2)] diagram of the fayalite-kirschsteinite-hedenbergite-magnetite-wollastonite (Fa-Kst-Hd-Mt-Wo) system at 400 degrees C and 1 kbar (Markl et al. 2001). Systematic thermodynamic analysis reveals that the composite exsolution lamellae of Mt and Hd in the olivine from the Ilimaussaq Intrusion, Greenland, should form when the reaction 3Fa+3Kst+O-2 = 3Hd+2Mt is overstepped on rapid cooling. This process is also accompanied by favorable f(O2) and a(SiO2) conditions. It is also found that the reaction mechanisms from Ca-Fe-rich olivine (Fa+Kst) to Mt+Hd are very different above and below the equilibrium temperature of the Mt-absent reaction Hd+Kst = Fa+2Wo. The mechanisms above the equilibrium temperature are obviously simpler than those below the equilibrium temperature. Despite this difference, the two types of mechanisms have many features in common: ( I) Mt and Hd can form in Ca-Fe-rich olivine on continuous cooling; (2) If a(SiO2) is not buffered, an increase in f(O2) alone is enough to transform Ca-Fe-rich olivine into Mt and Hd; (3) At a fixed L, or a(SiO2), the formation of Mt and/or Hd on cooling requires appropriate a(SiO2) or f(O2); and (4) The increase in a(SiO2) is favorable to the formation of lid, but it is not essential for the intergrown exsolution of Mt and lid. These common features are closely related to the fact that the process from Fa+Kst to Mt+Hd is an entropy decreasing oxidation reaction.
| Publication name |
AMERICAN MINERALOGIST Volume: 96 Issue: 4 Pages: 599-608 Published: APR 2011 |
| Author(s) |
Hu, Jiawen; Mao, Shide; Du, Guoqiang; Wu, Yunxia; Zhang, Pu |
| Corresponding author |
HU Jiawen
hu_jiawen@sina.com
1. Shijiazhuang Univ Econ, Coll Resources, Shijiazhuang 050031, Hebei Peoples R China
2. Chinese Acad Sci, Inst Geochem, State Key Lab Ore Deposit Geochem, Guiyang 550002, Guizhou Peoples R China |