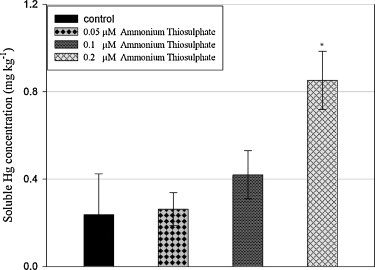
Fig. The concentration of mercury chemically solubilized by ammonium thiosulphate. Bars denote standard deviation from means of three replicates; significant differences among different molar ammonium thiosulphate solution are indicated by asterisks (p < 0.05).
According to the 'hard and soft' acid-base principle, mercury is a 'soft metal' and will preferentially form soluble chemical complexes with sulphur-containing ligands. In this work mercury uptake by Chenopodium glaucum L growing on mercury-contaminated soil was promoted using ammonium thiosulphate. The relative geochemical fractionation of mercury in the soil was subsequently investigated as a function of plant growth with and without thiosulphate amendment. The results indicate that the solubility of mercury is significantly increased through the application of thiosulphate to the soil. Substantially higher mercury levels were found in C. glaucum L treated with 2 g kg(-1) thiosulphate of soil when compared to the non-treated plants. Compared with initial soil, soluble and exchangeable fractions were increased both in planted and planted treated plants. However, no significant difference was observed between the soils of the planted and planted treated plants. The oxide-bound mercury concentration was significantly decreased for the planted soil (treated and non-treated) at the end of the experiment. Moreover, this fraction was highly correlated with the plant tissue mercury concentration. Taken together, thiosulphate assisted phytoextraction could be used to reduce environmental risk apparent for mercury-contaminated soil through reducing the oxide bound fractions, while managing the bioavailable fractions (compared with no treated plant). (C) 2010 Elsevier B.V. All rights reserved.
| Publication name |
JOURNAL OF HAZARDOUS MATERIALS Volume: 186 Issue: 1 Pages: 119-127 Published: FEB 15 2011 |
| Author(s) |
Wang, Jianxu; Feng, Xinbin; Anderson, Christopher W. N.; Qiu, Guangle; Ping, Li; Bao, Zhengduo |
| Corresponding author |
FENG XinBin
fengxinbin@vip.skleg.cn
Chinese Acad Sci, Inst Geochem, State Key Lab Environm Geochem, Guiyang 550002, Peoples R China |